Numbing for Aesthetic Procedures

Patient comfort should not be underestimated as a critical factor influencing the outcome and related patient satisfaction level associated with any cosmetic procedure, whether a botulinum toxin, filler, micro-needle, RF, fractional resurfacing laser or other pain-inducing superficial treatment. Therefore, numbing for aesthetics should be a careful consideration.
A painful aesthetic procedure is stressful for both physician and patient. Patients who perceive their experience as unreasonably or unnecessarily painful will, in all likelihood, neither return nor send referrals. As for the attending clinician, it may often be difficult to obtain a great technical injection on a patient who is clearly uncomfortable and too agitated to remain still during the injections or other aesthetic procedure.
Common Numbing Methods for Aesthetics
There are currently a variety of options available for numbing for aesthetics procedures. These options include topical preparations and injections as well as a few novel alternative treatments, such as chilling/cooling devices (among others), in which case the devices are often unreasonably costly as compared to current standards.
Topical anesthetics are being widely used in numerous medical and surgical sub-specialties including dermatology, podiatry, nephrology (dialysis cannulation), cardiology (heart catheterization), aesthetic dermatology and aesthetic surgery to name a few. They cause superficial loss of pain sensation after direct application. Their delivery and effectiveness can be enhanced by: increasing the drug concentration, lowering the melting point; by using physical and chemical permeation enhancers and lipid delivery vesicles. While using topical anesthetics, careful attention must be paid to their pharmacology, API concentration/dose, treatment surface area size, location and duration of application; age/weight of the patient and possible subject contraindicated pre-existing conditions.
Injections of local anesthetics are painful. It can worsen needle anxiety and can cause tissue edema, which distorts the treatment surface site. Using topical anesthesia allows clinicians to avoid all these problems associated with local anesthetic injections.
Compounded Numbing Topicals

Many clinicians in the aesthetic community have come accross compounded topical numbing products that have different concentrations of both amides and esters, primarily combinations of tetracaine, benzocaine, prilocaine and/or lidocaine.
Typically, compounded topicals are applied on patients for anywhere from 15 to 60 minutes. They are administered with or without occlusion (most often either Tegaderm or simple Saran Plastic Wrap) over the treatment area where applied. This process varies from practice to practice based on physician personal preference.
Two standardized compounded products seemed to have gained the most popularity regarding numbing for aesthetics:
- 20% benzocaine, 4% – 8% lidocaine and 2% – 4% tetracaine
- 7% – 10% of each of lidocaine and prilocaine or tetracaine with DMSO as the primary driver.
When applied, these compounded topical anesthetics produce a mild to moderate anesthesia in about 15 to 45 minutes. Variations of these formulae are also available.
DMSO in Numbing Creams
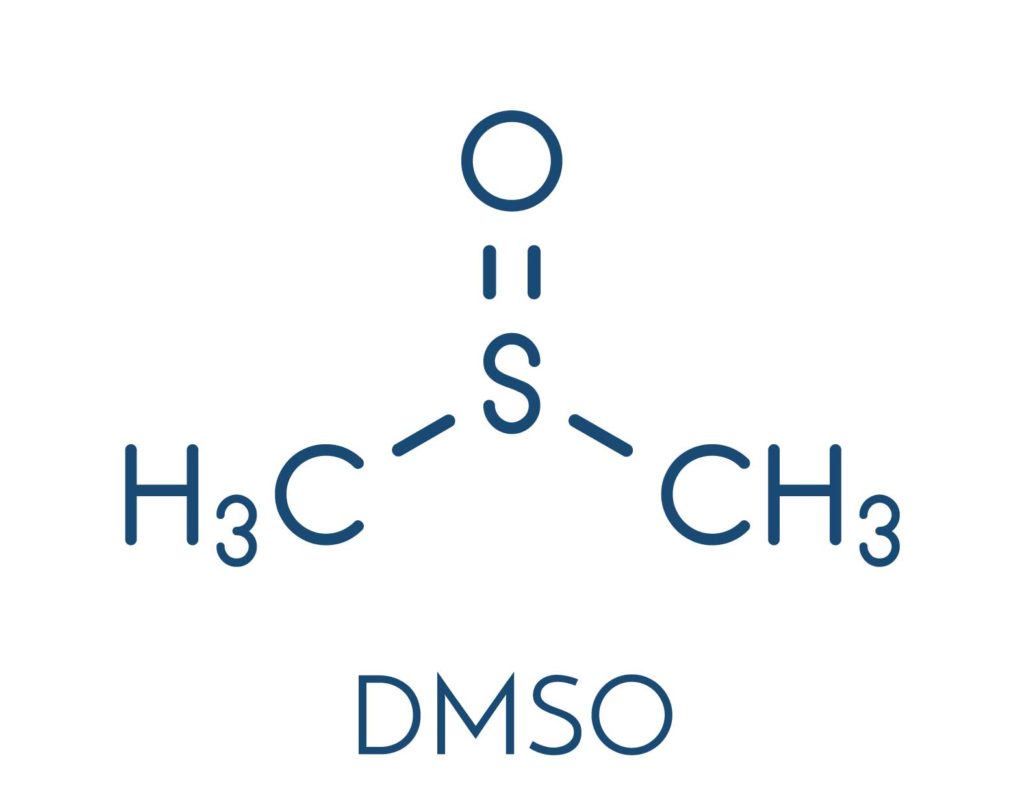
For the purposes of rapid delivery and onset, there is a fast growing trend among some compounding pharmacies to include a chemical driver called DMSO in their topical anesthetic formulations. DMSO is a very effective driver which seems to greatly reduce the time to initial onset of action. The only problem with DMSO is it also substantially increases introduction of the API into the circulatory system. Those experienced with the clinical pharmacology of topical anesthetics are aware that risk of adverse events increase as anesthetics enter the blood in increasing amounts and concentrations. Combining an already highly concentrated combination of anesthetic agents with DMSO logically carries with it a higher risk of adverse events as compared to safer or more prudently created alternatives (including other compounded products which do NOT contain DMSO).
Compound Numbing Cream Risks
Problems associated with the use of compounded topicals, which are not approved by the FDA, usually include their increased potential for toxicity, microbial and other contaminants or allergies due to variability in concentrations and sometimes less than standard cGMP’s, as well as liability associated with their lack of FDA approval.

Variability of Formulations
Unlike products that have been approved by the FDA or follow standard monograph policies, there is variability of the formulations depending on the pharmacy that produces the product and the level of experience and ability of the pharmacist who fills the order. Variability in some compounding pharmacies has resulted in a host of problems ranging from ineffective concentrations to those that are potentially harmful. A few documented deaths have been associated with the use of these agents.
 Toxicity
Toxicity
Toxicity from topical anesthetics (products used for numbing for aesthetics) may result in seizures and respiratory distress. If this does not instill a healthy respect for these topical medications, there have also been case reports of methemoglobinemia following administration of topical anesthetics in both pediatric and adult patients. For these reasons it is imperative that clinicians understand appropriate dosing standards as well as maximum safe total anesthetic dosing parameters and the mitigating factors that may lead to adverse events. Typically, most topical anesthetics are well tolerated when applied at appropriate concentrations/volumes, appropriate size treatment surface areas and in patients who do not have known contraindicated conditions such as severe renal or hepatic impairment or a familial predisposition to methemoglobinemia (a very rare condition affecting the oxygen carrying components in blood).
The toxic quantity required depends on the concentrations of the anesthetics contained. So as an example, unlike a 1% lidocaine with 1:100k of epinephrine injection, the amounts of product that are safe to use are somewhat variable and depend upon the manufacturing practices of the particular compounding pharmacy.
Avoiding product toxicity requires a knowledge of the recommended maximal amounts and the mathematical ability to convert the concentrations (often given as a percentage) to mg/kg. This latter point may sound trivial, but if asked, my personal experience with this concept is that most of your colleagues will probably need to refer to a textbook for the proper conversions. Toxic levels of the various anesthetics may be reached by applying large quantities of the drugs with high concentrations (and possibly included DMSO as the driver) onto large areas of the body.
For Prilocaine, the reported toxic level for dental use is 6 mg/kg. Lidocaine dosing should be limited to 3 mg/kg to 5 mg/kg when administered without

Liability
Whatever their origin or source, compounded products may be associated with liability issues regarding various state and federal regulations. Check with a local healthcare regulatory attorney or state Boards of Pharmacy to identify local regulations. No product is worth potential regulatory entanglements when effective alternatives are easily available.
Non Compounded Numbing Creams

Non compounded products — monograph compliant OTC lidocaine and associated products as well as FDA approved prescription products, provide the safety of having been certified by the FDA as safe and effective. In addition, there are no issues as with compounded products regarding distribution, crossing state lines by ordering from another state, or variability in manufactured concentrations.
There is limited safety data available referencing specific safe dosing parameters for the vast majority of OTC lidocaine and associated products. However, each of these are supplied in concentrations the FDA has classified as safe and effective. So long as these products are not abused, they have very limited history of serious adverse events when used as directed on small to medium size surface areas and to intact skin.
EMLA was the first topical medication approved for use as a topical anesthetic, and it still enjoys some limited use for numbing for aesthetics. However, it has largely been supplanted by more effective products. Its chief benefit is that it is safe and somewhat effective and it may be still possibly be covered by the patient’s insurance plan.
Improving Topical Anesthetic Efficacy
Contrary to popular belief the concentrations of a topical anesthetics’ active ingredients are not the sole factor in determining the efficacy of the product. There are several factors that will determine the efficacy of a topical anesthetic product, which include (among others):
Combinations of anesthetics
Some approved combinations create a ‘eutectic mixture’ with a lower melting point when combined than the melting point of the individual ingredients, which may tend to improve the absorption of the final product at normal skin temperature.
The type of delivery mechanism
The type delivery mechanism that is used is very important. A safe and effective delivery mechanism will assist in epidermal transference of the active ingredients. It is also important to carefully consider the nature and type of delivery mechanism and carrier if the product is to be used prior treatments that involve laser therapy. Obviously a carrier that may ignite (alcohols) or fry (oils) the skin during the application of laser are potentially very dangerous. Therefore, a topical anesthetic should be chosen on a purpose specific basis. A product that is suitable for one procedure may be dangerous if used for another type of procedure.
The pH
The final acidity/alkalinity of the anesthetic will not only affect the safety of the product it can also affect skin absorption.

The thickness/hardness/dryness of the skin
thicker, drier, or harder epidermal skin will take longer to penetrate. However the use of abrading devices prior to the application of topical anesthetics is contraindicated due to the potential to create raw skin that may increase deeper absorption and ultimately blood serum levels.
Natural patient skin oiliness and cleanliness
oily or otherwise obstructed skin may tend to repel anesthetic creams – it may be necessary to remove particulate matter, cosmetic products and natural oil from skin prior to applying a topical anesthetic for best outcome.
The duration of application of the product used for numbing for aesthetics procedures
too short a period and the anesthetic has not had time to work, too long and it may have worn off before the treatment commences. Different anesthetics will have a varied duration of peak efficacy therefore it is important to know the ‘Time Course’ (basically the onset and duration of action) of your products before using them.
The Time Course of the anesthetic product used for numbing for aesthetics procedures
in pharmacokinetics this is often referred to by the acronym LADME for the drug; Liberation – Absorption – Distribution – Metabolism – Excretion, with topical anesthetics the ‘Liberation’ & “Absorption’ time frames should be kept in mind. Some topical anesthetics are actually solids suspended in the carrier which only become liquids that are capable of absorption once they are applied to the skin and reach normal skin temperature (the melting point). In instances where a patient has just come from a cold environment the anesthetic used for numbing for aesthetics may seem to be less than adequately effective if sufficient time has not been allotted for the patient’s skin to warm up before the anesthetic is applied. Once absorption has occurred the distribution phase (achieving dermal anesthetization) for topical anesthetics will be rapid and there will be a window of peak efficacy for each individual product.
Application surface area
Smaller areas may actually be easier to achieve effective anesthesia than larger areas. If possible large areas should always be segmented and treated a small segment at a time in successive sessions with at least 24 hours between sessions, so as to reduce the risk of causing a toxic blood serum level that could occur when treating large area of skin even if you are using products containing low concentrations of anesthetic.

Pre/co-existing pain
Existing medical conditions that cause pain will have the tendency to increase sensitivity to any new sources of pain and the patient may also exhibit reduced efficacy for pain relief if they have been on long term analgesia.
Menstrual status (for female patients)
menstruating female patients may have a general heightened sensitivity to pain.
The mental state of the patient
feeling rushed, being being anxious, stressed or emotionally upset may all tend to heighten the client/patients sensitivity to pain.
The technique and manner of the technician
as with any procedure a technician who is both skilled and who projects calm confidence when interacting with the client/patient will help to reduce their overall anxiety about the intended procedure.
Indications for Aesthetic Numbing Products

Indications
For local analgesia on intact skin
Minimize discomfort prior to injections or before intravenous and arterial line
To relieve pruritus and pain due to minor burns, skin eruptions (e.g., herpes, sunburn, insect bites), stings, poison ivy, and minor cuts and scratches
For superficial dermatologic procedures like venipuncture, hair and warts removal, split thickness skin graft harvesting, shave or excision biopsy, dermabrasion for tattoo removal, venous leg ulcer debridement, curettage and electrosurgery, treatment of port-wine stains etc.
Contraindications
Ester group topical anesthetics are contraindicated in patients with known allergy to PABA, sulfonamides and hair dyes. It may also be prudent to exercise caution when administering either amide or ester anesthetics to patients with known severe renal and/or hepatic impairment due to reduced ability for the patient’s system to metabolize and clear the API or active metabolites, which may lead to cumulative toxicity, especially when applying those with very high API concentrations.
Patient Comfort During Aesthetic Procedures
Appropriate numbing for aesthetics procedures may make the difference between a happy cosmetic patient who returns for additional treatments and one who reconsiders the prospect of a visit to your office as a less than pleasant experience. Those competitors who provide as close to pain free or very limited pain experience combined with satisfactory procedural outcomes may be also be those who develop the best relationships over time.
The topical alternatives differ dramatically in their safety and efficacy. Compounded products offer the attraction of higher concentrations and the ability to formulate products that have specific ingredients. FDA-approved products offer the comfort of a process that has evaluated the product for safety and efficacy. Each has its own benefits worthy of consideration. All have a comparatively low cost of supply entry point as compared to the more expensive and possibly not as effective, bulky devices such as chiller devices as one example.

