Topical Anesthetic Definition
The basic topical anesthetic definition is: a cream, gel, spray, patch or lotion that is used to numb the surface of a body part. They can be used to numb any area of the skin as well as the front of the eyeball, the inside of the nose, ear, throat, the anus and/or the genital area. Examples include benzocaine, prilocaine, cocaine, lidocaine, pramoxine, and tetracaine. The topical anesthetic definition does not include analgesics such as menthol, camphor or capsaicin. These topical analgesics fall under the category, counter-irritants.
Topical anesthetics are supplied in many formats including: creams, gels, aqueous solutions, sprays and patches.
Another category of topical analgesics that does not fall under the topical anesthetic definition is the topical non-steroidal anti-inflammatory (NSAID’s), such as topical salicylate (essentially, aspirin) and acetaminophen. While anesthetics are analgesics, the strict topical anesthetic definition is a medication used to block neuronal impulses by interrupting the high voltage, sodium-ion channel that signals sensations of pain, burn and itch. Analgesics are simply medications that mitigate pain through one mechanism or another. Therefore, anesthetics fall under the category of analgesics, but not the other way around.
Topical Anesthetic Definition – Mechanism of Action
Active pharmaceutical ingredient, included in a local application form, that is administered to the skin, that alters signal conduction in neurons by blocking the fast voltage-gated Na+ channels in the neuronal cell membrane responsible for signal propagation. With sufficient blockage, the membrane of the postsynaptic neuron will not depolarize and will thus fail to transmit an action potential.
Uses of Topical Anesthetics:
Topical anesthetics are commonly used for the following:
- relieve pain, burn and itch sensations caused by minor skin irritation
- relieve pain and burn cause by minor skin injuries, such as, minor burns, scrapes and cuts
- relieve pain, burn and itch sensations caused by insect bites and stings
- mitigate pain caused by needle procedures, both medical and non- or quasi-medical, such as tattoos and piercings
- mitigate pain caused by minimally invasive medical and quasi-medical aesthetic procedures
- mitigate pain caused by suture administration or removal
- mitigate pain, burn and itch sensations caused by hemorrhoids
- mitigate sensations of pain, burn and itch caused by shingles (herpes zoster)
Topical Anesthetic Definition – Amino esters vs. amino amides:
Within the topical anesthetic definition there are two separate sub-classes. These sub-classes are: amino esters and amino benzoates.
Structurally, local anesthetics consist of three molecular components:
- a lipophilic part
- an intermediate aliphatic chain
- a hydrophilic (amine) part
The chemical linkage between the lipophilic part and the intermediate chain can be of the amide-type or the ester-type, and is the general basis for the current classification of local anesthetics.
Amino esters:
Amino esters, in reference to anesthetic agents, are rapidly metabolized in the plasma by butyrlcholinesterase to para-aminobenzoic acid derivatives, then excreted in the urine. This suggests their very short half lives. Allergy is more likely to occur with ester-type agents, as opposed to amide-type.
Examples of the common amino ester class are:
- benzocaine
- tetracaine
- procaine
- cocaine
Amino amides:
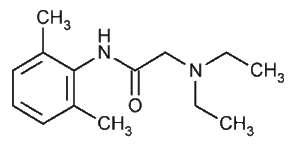
Amino amides have an amide link between the intermediate chain and the aromatic end, whereas amino esters have an ester link between the intermediate chain and the aromatic end. Amino amides are metabolized by the enzymatic process in the liver.
Common amino amides are:
- lidocaine
- prilocaine
- bupivacaine
Amino esters are unstable in solution, but amino amides are very stable in solution. Amino esters are much more likely than amino amides to cause allergic hypersensitivity reactions.